

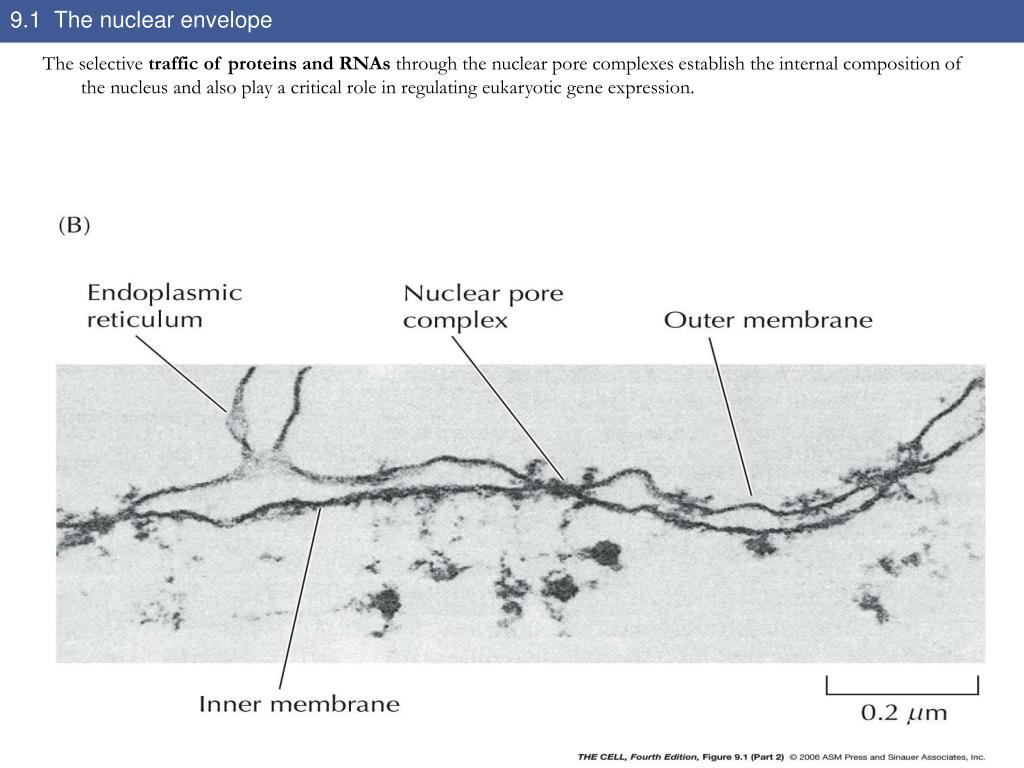
The energy released would be used to dissociate the cargo from the importins and to bind the cargo to the exportins. It has an outer unit membrane (thickness 7 nm phospholipid double layer) with attached ribosomes followed by the perinuclear space wich is 20 - 100 nm in. Viral capsids can escape out of the nucleus by disrupting the nuclear membrane envelope. Ran enzyme) help by hydrolyzing GTP ( guanosine triphosphate) so that energy would be released in the process. Nuclear transport needs energy to proceed. The traditional view of the nuclear envelope (NE) was that it represented a relatively inert physical barrier within the cell, whose main purpose. Also located at the nuclear envelope are nuclear pore complexes (. The nuclear membrane expands massively during closed mitosis. In contrast, the inner membrane has a distinct protein composition and specialized functions. On the other hand, the cargo binds with the exportin inside the nucleus, and then moved outside the nucleus via the nuclear pore. Nuclear envelope (NE) expansion must be controlled to maintain nuclear shape and function. On one hand, the cargo binds with the importin in the cytoplasm, and then moved into the nucleus through the nuclear pore. However, cargo proteins and RNAs that need to be transported require importins and exportins to enter and exit the nucleus, respectively. The nucleus is enclosed by a double membrane called the nuclear envelope (NE), which consists of the outer nuclear membrane (ONM), the inner nuclear membrane (INM), the nuclear pore membrane, and the embedded nuclear pore complexes (NPCs not reviewed here, for recent reviews see 1, 2 ). The nuclear membrane consists of two lipid bilayers enclosing the nucleus and physically isolating it from the rest of the cell, which enables important. Small molecules, such as ions, can pass through the nucleus with ease. Although the nuclear envelope is perforated with nuclear pores, large molecules would still need a nuclear transport mechanism in order to enter and exit the nucleus. proteins and RNA) into and out of the nucleus. The presence of the nuclear envelope prevents the easy passage of large molecules (e.g. It is postulated to occur via vesicle fusion or probably by reshaping of the endoplasmic reticulum, enclosing the nuclear region with a new nuclear envelope. The reformation process remains unclear how it proceeds. Then, during telophase, the nuclear membrane reforms. In animal and plant cells, the nuclear envelope breaks down into pieces during prometaphase of mitosis. An exception to this is the yeast cells whereby the nuclear envelope stays intact during cell division. Definition of nuclear envelope in the dictionary.
The nuclear envelope disintegrates to allow the spindle fibers to access the chromosomes in the nucleus. Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA (2006) A class of membrane proteins shaping the tubular endoplasmic reticulum.During cell division, the nuclear envelope undergoes major changes in animal and plant cells. Labels include nucleus, chloroplast, cytoplasm, membrane, cell wall, and vacuole. Spiliotis ET, Pentcheva T, and Edidin M (2002) Probing for membrane domains in the endoplasmic reticulum: Retention and degradation of unassembled MHC class I molecules. The nucleus is the organelle that controls the activities of the cell. Sitia R and Meldolesi J (1992) Endoplasmic reticulum - a dynamic patchwork of specialized subregions. Prunuske AJ and Ullman KS (2006) The nuclear envelope: form and reformation.

Pfeffer S (2003) Membrane domains in the secretory and endocytic pathways. Nikonov AY, Snapp E, Lippincott-Schwartz J, and Kreibich G (2002) Active translocon complexes labeled with GFP-Dad1 diffuse slowly as large polysome arrays in the endoplasmic reticulum. Nicchitta CV (2002) A platform for compartmentalized protein synthesis: protein translation and translocation in the ER. Like/Share & Subscribe to our YouTube channel for more such learning conceptsConcepts of the video: 0:00 - 0:36 : About the ultra structure. Ilnuma T, Aoki T, Arasaki K, Hirose H, Yamamoto A, Samata R, Hauri H, Arimitsu M, Tagaya M, Tani K (2009) Role of syntaxin 18 in the organization of endoplasmic reticulum subdomains. Int Rev Cytol 205: 149ĭeschuyteneer M, Eckhardt AE, Roth J, and Hill RL (1988) The subcellular localization of apomucin and nonreducing terminal N-acetylgalactosamine in porcine submaxillar glands. Baumann O and Walz B (2001) Endoplasmic reticulum of animal cells and its organization into structural and functional domains.


 0 kommentar(er)
0 kommentar(er)
